Introduction to Quantum Physics
PUBLISHED: Jul 11, 2024
An introduction to the world of quantum physics.
Contents
Quantum physics stands at the beginning of the 20th century - with great minds from Planck to Hilbert navigating the complex world of quantum physics.
We begin with a conceptual understanding of the field—moving down from our classical world (or should
we say moving smaller?).
I. The Birth of Quantum Theory
Quantum theory goes hand-in-hand with the emergence of the Bohr model in atomic theory, and the initiation of statistical mechanics by Boltzmann, both conceptually and chronologically. To avoid meandering, we’ll provide only the necessary bits from both of these conceptual bodies, but it’s very useful to have a good understanding of both when going into quantum theory!
1. Blackbodies and Energy Quantization
A blackbody is an ideal object that absorbs all electromagnetic waves, regardless of the wave frequency and angle of incidence. Blackbody radiation is the radiation emitted by a blackbody in thermal equilibrium with its environment.
A classic example of a blackbody is a cavity, such as a box, with thermally insulated walls with a small hole:
If an electromagnetic ray “hits” the hole, it will enter the insulated blackbody and will be reflected inside, with energy transferring between the cavity and the light, before it exits the cavity by chance. This energy transfer enables the light to reach thermal equilibrium with the cavity itself—making the light that exits the blackbody “different” from the initial ray.
1.1 The Physics of the Blackbody
To understand the significance of the blackbody to quantum theory, we will first investigate the physics of this blackbody with statistical mechanics—particularly, the energy of the wave.
First, consider a cubic cavity with side lengths , and a small hole to allow a trapped electromagnetic wave to escape. Given that an electromagnetic wave has been trapped in this blackbody, we establish:
- The electric field at the walls must be .
The walls of our blackbody are conductive, so at these boundaries.- The trapped wave can be represented as a superposition of sinusoidal waves.
With (1) established, we know that the Fourier expansion of this wave is complete.- The modes of standing waves are dimensionally-independent.
An extension of (2), this simply states that the standing wave modes that we use to represent our trapped wave in each direction are independent of each other—for example, any mode in the direction can exist with any mode in the or direction.
Opting to represent all possible modes of this wave mathematically,
where n is an integer. Since we have 3 degrees of freedom with the same side length , we have 3 separate
for each degree of freedom: .
How many different configurations of n can we have? Well, we know can be any positive integer, and we have
3 different for each degree of freedom. The total amount of different configurations of we can have
would be:
This isn’t very useful of an expression, so we instead consider the volume of a coordinate system—the above expression is the same as the volume of the positive octant of a cartesian coordinate system! So to represent all possible wave modes:
We integrate in the positive octant of a coordinate system . The factor of 2 that we multiply
this integral by comes from the fact that all light has two polarization modes which doubles the
possible configurations of .
However, we want to represent the total modes in terms of a more typical quantity—angular frequency. To do this,
we take a few steps:
Where represents the wavenumber, and represents the angular frequency of this wave. Then, converting the above expression:
Where volume . With this representation, we use the equipartition theorem to state the energy of this wave:
1.2 Planck and The Ultraviolet Catastrophe
Physicists in the early 20th century came to the same conclusion as above to represent the energy of a blackbody. To follow, the formulated Rayleigh-Jeans Law takes the above expression with intensity formula to state:
which makes , as all other variables are constants. This means, at high angular frequencies, our intensity increases rapidly without bound—which is known as the Ultraviolet Catastrophe.
We know this relation is untrue, due to the fact that the energy inside the blackbody is finite, while the expression says that at infinitely high frequencies, the blackbody will emit an infinite amount of radiation and energy.
Planck noted this too; the square-relation of spectral intensity to angular frequency meant that our current model resulting in the UV catastrophe was incorrect. He instead developed a model using the assumption that radiation emitted a whole number multiple of finite packets of energy, dubbed quanta.
In the case of our blackbody and light, this packet of energy was discovered 5 years later by Einstein and named—the photon. The energy of these packets is dictated by
With this, we modify our relation to create a new partition function to find an expression for intensity.
Formulating a new expression for energy
Recall from 1.1 thatwhen finding the total number of modes for our blackbody. Given this, we formulate our partition function:
We want to transform this into an expression for our energy expectation value to use our earlier expression :
Finally performing the partial derivative,
With this expression for energy, we arrive at Planck’s law:
Notice we used the same energy-intensity relation from before. This equation can be manipulated very simply converting angular frequency to frequency and substituting constants: , and into a more recognizable form:
Note that the volume drops out, as is an expression of spectral intensity per unit.
What does this mean for our blackbody? Well, the intensity of the radiation emitted by the blackbody is now finite at all frequencies, and the UV catastrophe is avoided. This is the first step into the world of quantum physics—the quantization of energy.
We have established that energy is discrete, coming in integer multiples of packets, kind of like individual balls of energy.
2. The Wave-Particle Duality
Having established that energy is quantized, we move forward to the next fundamental concept of quantum mechanics—the wave-particle duality.
It’s important to understand what we mean by wave-like and particle-like behavior in the context of physics:
- Particle-like behavior describes behavior that obeys classical mechanics, the motion of everyday objects such as a box or a ball as we understand it. These classical particles have definite positions and momenta, and obey classical concepts such as Newton’s laws. Most importantly, particles do not exhibit interference - they do not occupy the same space as each other and interfere.
- Wave-like behavior describes behavior modeled by the wave equation. Classical waves display interference when multiple waves occupy the same space, and exhibit other behaviors such as diffraction.
These behaviors are both models for the behaviors of physical things, and in the classical world, they are largely mutually exclusive—that is, something like sound is modeled by a wave, while a baseball is modeled by a particle.
However, the lines begin to blur in the quantum scope. This was first observed in the behavior of light, but as we’ll come to see, this blurring applies to all matter.
2.1 The Photoelectric Effect
The photoelectric effect is a demonstration of light’s behavior as a particle.
2.2 The Double-Slit Experiment
2.3 The Matter Wave
II. Fundamental Quantum Concepts
Having established our theoretical precendent for quantum mechanics, we now move to explaining the concepts of quantum mechanics.
1. The Language of Quantum Mechanics
The language of quantum mechanics can be simplified to two basic fundamental concepts: wave functions, and observables.
- A wave function describes the state of a system—all of its properties at a given time. Essentially, when discussing the state of something, we are discussing everything we know about it at a given time. For example, the state of a moving particle would include its position and momentum.
- An observable is a property of a system that can be measured.
1.1 Wavefunctions
The wavefunction is a mathematical function that describes the quantum state of a given system. Much like how a physicist might use or determine a function for an object’s position in a classical scenario, a wavefunction represents the state of a system—for example, a particle.
This function is complex-valued and satisfies the definition of a vector in the infinite-dimensional Hilbert space. As such, we use linear algebra and vector properties to manipulate wavefunctions to yield information about the system.
Properties
Let’s consider the previously given wavefunction . At first glance, the function’s physical meaning is difficult to interpret; it gives a function for any time .
So, what does the wavefunction represent? We know from our previous theoretical discussions that matter does not behave explicitly like a particle at the quantum scale; systems are not localized at a single point in space. To answer this, we turn to Born’s interpretation of the wavefunction:
We see that the integral of the wavefunction squared over a given interval gives the probability of finding the particle within that interval at a given time.
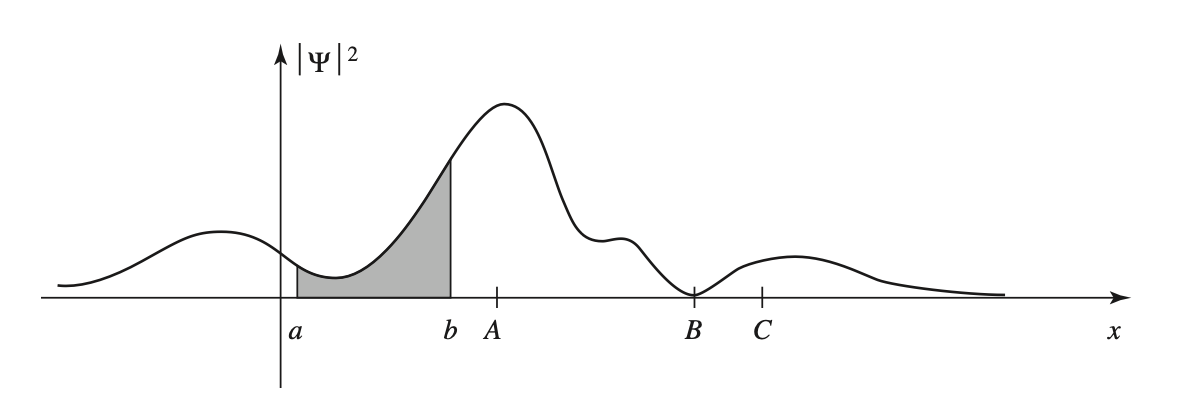
image from Griffiths.
Basic mathematical properties of the wavefunction
- The wavefunction is represented in bra-ket notation as .
- The wavefunction vector is a sum of linearly independent vectors. This is a linear combination of eigenstates of the system with coefficients :
- The wavefunction is normalized:
Note that the last point shows the integral of an already normalized wavefunction. You may encounter wavefunctions that have the integral equal a constant; we then use a fitting multiplicative constant and multiply the wavefunction accordingly to normalize it. As we’ll see in later sections, this multiplication is valid, as the wavefunction is a solution to a differential equation and thus can be multiplied by a constant and remain in the solution space.
Then, what about this case?
If the integral is infinite or , we state that the wavefunction is not normalizable, as there is no constant value that can be multiplied to normalize the integral. Thus, these wavefunctions can not be physically realizable are rejected as solutions.
Based on Born’s interpretation and vector properties of the wavefunction, we now know how to find the probability of a system being in a certain state. By using the orthonormality of eigenstates:
Probability of an eigenstate
1.2 Observables and Measurement
An observable is a property of a system that can be measured. In quantum mechanics, observables are represented by Hermitian operators. While wavefunctions describe the state of a particle, observables are the properties that are measured.
We use operators to determine
Basic properties of operators
- Operators are represented in bra-ket notation as .
- Operators will always have real eigenvalues, as they are hermitian; these eigenvalues correspond to measureable quantities.
In other words, when we make a measurement, the result will correspond to an eigenvalue of the operator.
- For an eigenvalue to represent a physically measureable outcome, the associated eigenvector must lie in the Hilbert space.
- Not all physical observables are associated with non-trivial hermitian operators.
2. The Schrödinger Equation
The Time-Dependent Schrödinger Equation (TDSE)